Influenza (or “flu”) is a respiratory infection caused by an influenza virus. Influenza outbreaks happen every year.
Influenza (flu) vaccination is safe for anyone 6 months of age and older. It protects you and those around you from the flu and its complications.


Because influenza viruses change – often from year to year – people don’t stay immune for very long. While there may be influenza cases in the country all year round the disease shows two peaks – a major peak during winter (January to March) and a significant peak in the post-monsoon season of August to October. Flu shots are usually given once a year. The shots provide protection throughout the flu season.
- Babies and children 6 months to 9 years of age who have never had a flu shot will need 2 doses of the vaccine, given at least 4 weeks apart.
- Children who had one or more doses of the regular seasonal flu shot in the past will only need one dose per year.
Our discussion points are:
- Should My Child Get an Influenza (Flu) Shot? (High-risk groups)
- What is Influenza (Flu)?
- Types of Influenza (Flu) Strains
- How Does Influenza (Flu) Spread?
- Clinical Features in Influenza (Flu)
- Cold vs Influenza (Flu)
- Similarities & Differences Between COVID-19 & Influenza (Flu)
- Influenza (Flu) Complications
- Different Types of Influenza (Flu) Vaccines
- Influenza (Flu) Vaccine Benefits
- Influenza (Flu) Vaccine Match (FAQs)
- Influenza (Flu) Vaccine Side Effects
- Conclusion
Should my child get a Influenza (Flu) Shot?
Universal Recommendation:
All children aged 6 months and older should get a flu shot every year to protect themselves and those around them from the complications of influenza.


High-Risk Groups for Influenza Complications:
The vaccine is particularly important for children and teenagers at high risk of severe flu complications, including those who:
- Are between 6 months and 8 years of age.
- Have chronic heart or lung disorders, such as bronchopulmonary dysplasia, cystic fibrosis, or asthma requiring regular medical follow-up.
- Have chronic conditions that weaken the immune system, such as immune deficiencies, cancer, or HIV, or are on treatments causing immune suppression.
- Have diabetes or other metabolic diseases.
- Have chronic kidney disease.
- Have chronic anemia or a hemoglobin disorder.
- Have a chronic neurological disorder.
- Are severely obese (body mass index ≥40).
- Are pregnant.
- Take acetylsalicylic acid (ASA or Aspirin) daily.
- Live in a chronic care facility.
- Live with someone at high risk of flu complications (e.g., another child or adult with the above conditions).
Children Under 8 Years Old:
Children younger than 8 years old are at a higher risk of severe complications from the flu, including high fever, convulsions, and pneumonia. If your child falls into this age group or has underlying health conditions, it is crucial to ensure everyone in the household gets vaccinated to provide an added layer of protection.
Special Considerations for Caregivers:
Caregivers of children younger than 8 years of age should also be immunized. This is especially critical if you have:
- Children younger than 6 months old, who are too young to receive the flu vaccine.
- A pregnant member in the household.
What is Influenza (Flu)?
The flu, or influenza, is a viral respiratory infection that spreads from person to person through secretions from the nose and lungs, such as when sneezing. Unlike other respiratory infections often categorized as URIs (upper respiratory infections), influenza causes higher fever, severe body aches, and more malaise. People often mistakenly refer to these other infections as “flu,” but they are caused by different viruses and are less severe.
Types of Influenza Viruses
Influenza viruses are classified into three main types: A, B, and C:
- Type A: Responsible for most epidemics and pandemics. These viruses are further divided into subtypes based on two surface proteins: hemagglutinin (H) and neuraminidase (N). There are 16 known H subtypes and 9 N subtypes.
- Type B: Causes seasonal outbreaks but is less severe than Type A.
- Type C: Usually leads to mild respiratory illness or no symptoms and does not cause epidemics.
Historical Insights: The 2009 Swine Flu Pandemic
The 2009 swine flu pandemic was caused by a novel influenza A virus, H1N1, named for its surface protein types. Although initially linked to pigs because of its genetic similarity to swine influenza viruses, this virus was distinct from typical swine flu viruses.
The Impact of Influenza
The flu is a common illness with significant public health implications. For example, during the 2018/2019 influenza season in the United States:
- 5%-20% of the population contracted the flu.
- Nearly 500,000 people were hospitalized due to flu-related complications.
- About 34,000 people died from the flu or its complications.
Influenza types A and B are the primary drivers of these seasonal epidemics, underlining the importance of prevention and treatment.
Types of Influenza Viruses
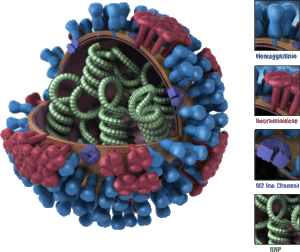

Influenza Virus Types
There are four types of influenza viruses: A, B, C, and D.
- Human influenza A and B viruses: Cause seasonal flu epidemics (flu season) almost every winter.
- Influenza A viruses: The only influenza viruses known to cause flu pandemics (global epidemics of flu disease).
- Pandemics occur when a new and distinct influenza A virus emerges, infects humans, and spreads efficiently.
- Influenza C viruses: Cause mild illness and are not associated with flu epidemics.
- Influenza D viruses: Primarily affect cattle and are not known to infect humans or cause illness.
Influenza A Subtypes
Influenza A viruses are further classified into subtypes based on the properties of two surface proteins:
- Hemagglutinin (H): 18 subtypes (H1 through H18).
- Neuraminidase (N): 11 subtypes (N1 through N11).
By combining these proteins, influenza A subtypes are named (e.g., A(H1N1), A(H3N2)).
- Current circulating subtypes in humans: A(H1N1) and A(H3N2).
- Potential diversity: While 198 subtype combinations are possible, only 131 subtypes have been detected in nature.
Influenza A subtypes can also be divided into genetic clades and sub-clades for more specific classification.
Visual Representation
The following is an image representing the influenza virus structure:
The hemagglutinin (H) and neuraminidase (N) proteins are essential in determining the subtype of the virus and play a significant role in its ability to infect humans and animals.
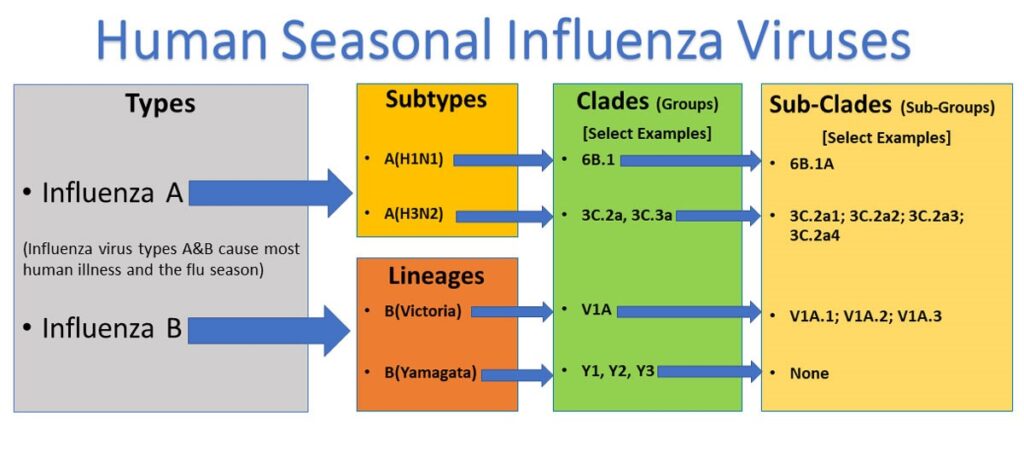

Classification of Influenza Viruses
The two types of influenza viruses responsible for most human illnesses and seasonal flu each year are:
- Influenza A viruses: Classified into subtypes based on their surface proteins (e.g., H1N1, H3N2).
- Influenza B viruses: Divided into two lineages: B/Yamagata and B/Victoria.
Both influenza A and B viruses can be further subdivided into clades and sub-clades, sometimes referred to as groups and sub-groups.
What Are Clades and Sub-Clades?
Clades and sub-clades represent further subdivisions of influenza viruses based on the similarity of their hemagglutinin (HA) gene sequences.
- Clade or group: A higher-level classification showing related viruses with similar genetic changes.
- Sub-clade or sub-group: A more specific subdivision within a clade.
These classifications allow researchers to track the evolution of influenza viruses and monitor the spread of specific genetic variants.
Phylogenetic Tree Representation
A phylogenetic tree groups related viruses together on branches based on genetic similarity.
- Nodes: Represent a single common ancestor of the viruses within a clade or sub-clade.
- Branches: Indicate how viruses are genetically related.
In above diagram, influenza viruses with shared genetic changes (e.g., nucleotide or amino acid changes) are grouped into clades and sub-clades, enabling flu experts to track which genetic groups are circulating in a population.
History of Influenza
Currently circulating influenza A(H1N1) viruses are related to the pandemic 2009 H1N1 virus that emerged in the spring of 2009 and caused a flu pandemic (CDC 2009 H1N1 Flu website). This virus scientifically called the “A(H1N1)pdm09 virus,” and more generally called “2009 H1N1,” has continued to circulate seasonally since then. These H1N1 viruses have undergone relatively small genetic changes and changes to their antigenic properties (i.e., the properties of the virus that affect immunity) over time.


Of all the influenza viruses that routinely circulate and cause illness in people, influenza A(H3N2) viruses tend to change more rapidly, both genetically and antigenically. Influenza A(H3N2) viruses have formed many separate, genetically different clades in recent years that continue to co-circulate.
Influenza B viruses are not divided into subtypes but instead are further classified into two lineages: B/Yamagata and B/Victoria. Similar to influenza A viruses, influenza B viruses can then be further classified into specific clades and sub-clades. Influenza B viruses generally change more slowly in terms of their genetic and antigenic properties than influenza A viruses, especially influenza A(H3N2) viruses. Influenza surveillance data from recent years shows the co-circulation of influenza B viruses from both lineages around the world. However, the proportion of influenza B viruses from each lineage that circulates can vary by geographic location.
Naming of Influenza Viruses
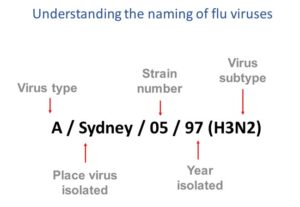

This image shows how influenza viruses are named. The name starts with the virus type, followed by the place the virus was isolated, followed by the virus strain number, the year isolated, and finally, the virus subtype.
Naming Influenza Viruses
CDC follows an internationally accepted naming convention for influenza viruses. This convention was accepted by WHO in 1979 and published in February 1980 in the Bulletin of the World Health Organization, 58(4):585-591 (1980) (see A revision of the system of nomenclature for influenza viruses: a WHO Memorandum pdf icon[854 KB, 7 pages]). The approach uses the following components:
- The antigenic type (e.g., A, B, C, D)
- The host of origin (e.g., swine, equine, chicken, etc.). For human-origin viruses, no-host of origin designation is given. Note the following examples:
- (Duck example): avian influenza A(H1N1), A/duck/Alberta/35/76
- (Human example): seasonal influenza A(H3N2), A/Perth/16/2019
- Geographical origin (e.g., Denver, Taiwan, etc.)
- Strain number (e.g., 7, 15, etc.)
- Year of collection (e.g., 57, 2009, etc.)
- For influenza A viruses, the hemagglutinin, and neuraminidase antigen descriptions are provided in parentheses (e.g., influenza A(H1N1) virus, influenza A(H5N1) virus)
- The 2009 pandemic virus was assigned a distinct name: A(H1N1)pdm09 to distinguish it from the seasonal influenza A(H1N1) viruses that circulated prior to the pandemic.
- When humans are infected with influenza viruses that normally circulate in swine (pigs), these viruses are call variant viruses and are designated with a letter ‘v’ (e.g., an A(H3N2)v virus).
Influenza Vaccine Viruses
One influenza A(H1N1), one influenza A(H3N2), and one or two influenza B viruses (depending on the vaccine) are included in each season’s influenza vaccines. Getting a flu vaccine can protect against flu viruses that are like the viruses used to make vaccine. Information about this season’s vaccine can be found at Preventing Seasonal Flu with Vaccination. Seasonal flu vaccines do not protect against influenza C or D viruses. In addition, flu vaccines will NOT protect against infection and illness caused by other viruses that also can cause influenza-like symptoms. There are many other viruses besides influenza that can result in influenza-like illness (ILI) that spread during flu season.
How does Influenza (Flu) Spreads?


Person to Person
People with flu can spread it to others up to about 6 feet away. Most experts think that flu viruses are spread mainly by droplets made when people with flu cough, sneeze, or talk. These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs. Less often, a person might get flu by touching a surface or object that has flu virus on it and then touching their own mouth, nose, or possibly their eyes.
When Flu Spreads
People with flu are most contagious in the first three to four days after their illness begins. Most healthy adults may be able to infect others beginning 1 day before symptoms develop and up to 5 to 7 days after becoming sick. Children and some people with weakened immune systems may pass the virus for longer than 7 days.
Symptoms can begin about 2 days (but can range from 1 to 4 days) after the virus enters the body. That means that you may be able to pass on the flu to someone else before you know you are sick, as well as while you are sick. Some people can be infected with the flu virus but have no symptoms. During this time, those people may still spread the virus to others.
Period of Contagiousness
You may be able to pass on the flu to someone else before you know you are sick, as well as while you are sick.
- People with flu are most contagious in the first 3-4 days after their illness begins.
- Some otherwise healthy adults may be able to infect others beginning 1 day before symptoms develop and up to 5 to 7 days after becoming sick.
- Some people, especially young children and people with weakened immune systems might be able to infect others with flu viruses for an even longer time.
Clinical Features in Influenza (Flu)
What are the emergency warning signs of flu?
People experiencing these warning signs should obtain medical care right away.
In children
- Fast breathing or trouble breathing
- Bluish lips or face
- Ribs pulling in with each breath
- Chest pain
- Severe muscle pain (child refuses to walk)
- Dehydration (no urine for 8 hours, dry mouth, no tears when crying)
- Not alert or interacting when awake
- Seizures
- Fever above 104°F
- In children less than 12 weeks, any fever
- Fever or cough that improves but then returns or worsen
- Worsening of chronic medical conditions
In adults
- Difficulty breathing or shortness of breath
- Persistent pain or pressure in the chest or abdomen
- Persistent dizziness, confusion, inability to arouse
- Seizures
- Not urinating
- Severe muscle pain
- Severe weakness or unsteadiness
- Fever or cough that improves but then returns or worsen
- Worsening of chronic medical conditions
These lists are not all-inclusive. Please consult your medical provider for any other symptom that is severe or concerning.
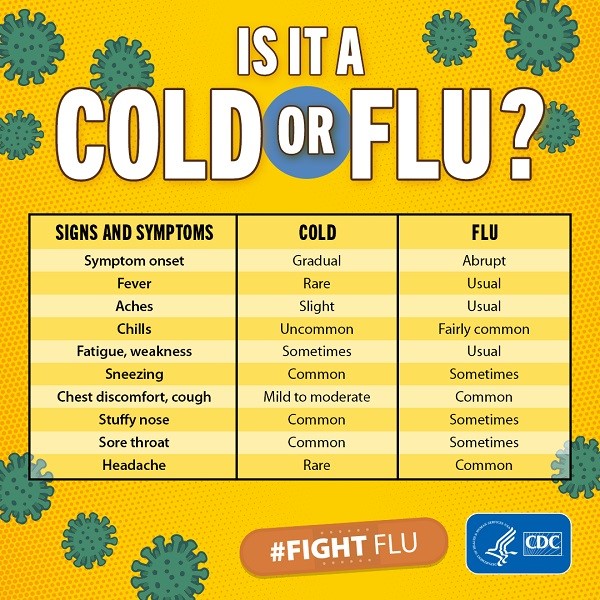
Cold vs Flu
What is the difference between a cold and flu?
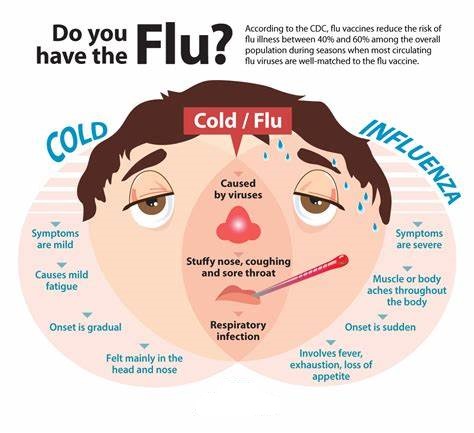

Flu and the common cold are both respiratory illnesses but they are caused by different viruses. Because these two types of illnesses have similar symptoms, it can be difficult to tell the difference between them based on symptoms alone. In general, flu is worse than the common cold, and symptoms are more intense. Colds are usually milder than flu. People with colds are more likely to have a runny or stuffy nose. Colds generally do not result in serious health problems, such as pneumonia, bacterial infections, or hospitalizations. Flu can have very serious associated complications.
How can you tell the difference between a cold and the flu?
Because colds and flu share many symptoms, it can be difficult (or even impossible) to tell the difference between them based on symptoms alone. Special tests that usually must be done within the first few days of illness can tell if a person has the flu.
What are the symptoms of the flu versus the symptoms of a cold?
The symptoms of flu can include fever or feeling feverish/chills, cough, sore throat, runny or stuffy nose, muscle or body aches, headaches, and fatigue (tiredness). Cold symptoms are usually milder than the symptoms of flu. People with colds are more likely to have a runny or stuffy nose. Colds generally do not result in serious health problems.

Covid19 vs Flu
What is the difference between Influenza (Flu) and COVID-19?
Influenza (flu) and COVID-19 are both contagious respiratory illnesses, but they are caused by different viruses. COVID-19 is caused by infection with a coronavirus first identified in 2019, and flu is caused by infection with influenza viruses.
COVID-19 seems to spread more easily than the flu. However, as more people become fully vaccinated against COVID-19, the spread of the virus that causes COVID-19 should slow down. More information about COVID-19 vaccines and how well they work.
Compared to flu, COVID-19 can cause more serious illnesses in some people. COVID-19 can also take longer before people show symptoms and people can be contagious for longer. More information about the differences between flu and COVID-19 is available in the different sections below.
Because some of the symptoms of flu, COVID-19, and other respiratory illnesses are similar, the difference between them cannot be made based on symptoms alone. Testing is needed to tell what the illness is and to confirm a diagnosis. People can be infected with both flu and the virus that causes COVID-19 at the same time and have symptoms of both influenza and COVID-19.
While more is learned every day about COVID-19 and the virus that causes it, there are still things, such as post-COVID conditions, that are unknown. This page compares COVID-19 and flu, given the best available information to date.
Signs & Symptoms of Covid19 & Flu
Similarities:
Both COVID-19 and flu can have varying degrees of signs and symptoms, ranging from no symptoms (asymptomatic) to severe symptoms. Common symptoms that COVID-19 and flu share include:

- Fever or feeling feverish/having chills
- Cough
- Shortness of breath or difficulty breathing
- Fatigue (tiredness)
- Sore throat
- Runny or stuffy nose
- Muscle pain or body aches
- Headache
- Vomiting and diarrhea
- Change in or loss of taste or smell, although this is more frequent with COVID-19.
How Long Symptoms Appear After Exposure and Infection in COVID19 & FLU?
Similarities:
For both COVID-19 and flu, 1 or more days can pass between when a person becomes infected and when he or she starts to experience illness symptoms.
Differences:
If a person has COVID-19, it could take them longer to experience symptoms than if they had flu.
Flu – Typically, a person experiences symptoms anywhere from 1 to 4 days after infection.
COVID-19 – Typically, a person experiences symptoms about 5 days after being infected, but symptoms can appear 2 to 14 days after in
How Long Someone Can Spread the Virus of COVID19 & FLU?
Similarities:
For both COVID-19 and flu, it’s possible to spread the virus for at least 1 day before experiencing any symptoms.
Differences:
If a person has COVID-19, they could be contagious for a longer time than if they had flu.
Flu
Most people with flu are contagious for about 1 day before they show symptoms.
Older children and adults with flu appear to be most contagious during the initial 3-4 days of their illness but many people remain contagious for about 7 days.
Infants and people with weakened immune systems can be contagious for even longer.
COVID-19
How long someone can spread the virus that causes COVID-19 is still under investigation.
It’s possible for people to spread the virus for about 2 days before experiencing signs or symptoms (or possibly earlier) and remain contagious for at least 10 days after signs or symptoms first appeared. If someone is asymptomatic or their symptoms go away, it’s possible to remain contagious for at least 10 days after testing positive for COVID-19. People who are hospitalized with severe disease and people with weakened immune systems can be contagious for 20 days or longer.
How INFLUENZA or COVID19 Spreads?
Similarities:
Both COVID-19 and flu can spread from person to person between people who are in close contact with one another (within about 6 feet). Both are spread mainly by large and small particles containing viruses that are expelled when people with the illness (COVID-19 or flu) cough, sneeze or talk. These particles can land in the mouths or noses of people who are nearby and possibly be inhaled into the lungs. In some circumstances, such as indoor settings with poor ventilation, small particles might be spread further than 6 feet and cause infections.
Although most spread is by inhalation, it may be possible that a person can get infected by touching (for example, shaking hands with someone who has the virus on their hands) or by touching a surface or object that has the virus on it, and then touching their own mouth, nose, or eyes.
Both flu viruses and the virus that causes COVID-19 can be spread to others by people before they begin showing symptoms; by people with very mild symptoms; and by people who never experience symptoms (asymptomatic people).
Differences:
While the virus that causes COVID-19 and flu viruses are thought to spread in similar ways, the virus that causes COVID-19 is generally more contagious than flu viruses. Also, COVID-19 has been observed to have more superspreading events than flu. This means the virus that causes COVID-19 can quickly and easily spread to a lot of people and result in continual spreading among people as time progresses.
People at High-Risk for Severe Illness of COVID19 & FLU
Similarities:
Both COVID-19 and flu illness can result in severe illness and complications. Those at highest risk include:
- Older adults
- People with certain underlying medical conditions (including infants and children)
- Pregnant people
Differences:
Overall, COVID-19 seems to cause more serious illnesses in some people.
For young children, especially children younger than 5 years old, the risk of serious complications is higher for flu compared with COVID-19. However, serious COVID-19 illness resulting in hospitalization and death can occur even in healthy young children.
Compared to young children, teens and adolescents with COVID-19 are more likely to have Multisystem Inflammatory Syndrome in Children (MIS-C), a rare but severe complication of COVID-19. However, for adolescents, the risk of serious COVID-19 illness is less than in children younger than 5.
Complications in COVID19 & FLU
Similarities:
Both COVID-19 and flu can result in complications, including:
- Pneumonia
- Respiratory failure
- Acute respiratory distress syndrome (fluid in the lungs)
- Sepsis (a life-threatening illness caused by the body’s extreme response to an infection)
- Cardiac injury (for example, heart attacks and stroke)
- Multiple-organ failure (respiratory failure, kidney failure, shock)
- Worsening of chronic medical conditions (involving the lungs, heart, or nervous system or diabetes)
- Inflammation of the heart, brain, or muscle tissues
- Secondary infections (bacterial or fungal infections that can occur in people who have already been infected with flu or COVID-19)
Differences:
Flu
Most people who get flu will recover on their own in a few days to two weeks, but some people will experience severe complications, requiring hospitalization. Some of these complications are listed above. Secondary bacterial infections are more common with influenza than with COVID-19.
Diarrhea is more common in young children with flu than in adults with flu.
COVID-19
Additional complications associated with COVID-19 can include:
- Blood clots in the veins and arteries of the lungs, heart, legs or brain
- Multisystem Inflammatory Syndrome in Children (MIS-C) and in Adults (MIS-A)
Long COVID is a range of symptoms that can last weeks or months after first being infected with the virus that causes COVID-19 or can appear weeks after infection. Long COVID can happen to anyone who has had COVID-19, even if their illness was mild, or if they had no symptoms.
Approved Treatments for COVID19 & FLU
Similarities:
People at higher risk of complications or who have been hospitalized for COVID-19 or flu should receive supportive medical care to help relieve symptoms and complications.
Differences:
Flu
Prescription influenza antiviral drugs are FDA-approved to treat flu-like Oseltamivir.
People who are hospitalized with flu or who are at increased risk of complications and have flu symptoms are recommended to be treated with antiviral drugs as soon as possible after illness onset.
COVID-19
The National Institutes of Health (NIH) has developed guidance on treatment of COVID-19, which is regularly updated as new evidence on treatment options emerges.
Vaccine for COVID19 & FLU
Similarities:
Vaccines for COVID-19 and flu are approved and/or authorized for emergency use (EUA) by FDA.
Differences:
Flu
There are multiple FDA-licensed influenza vaccines produced annually to protect against the 4 flu viruses that scientists expect will circulate each year.
COVID-19
Three COVID-19 vaccines have been authorized for use by FDA under an EUA. Other vaccines to prevent COVID-19 are under development.
Influenza (Flu) Complications
Most people who get flu will recover in a few days to less than two weeks, but some people will develop complications (such as pneumonia) as a result of flu, some of which can be life-threatening and result in death.
Sinus and ear infections are examples of moderate complications from flu, while pneumonia is a serious flu complication that can result from either influenza virus infection alone or from co-infection of flu virus and bacteria. Other possible serious complications triggered by flu can include inflammation of the heart (myocarditis), brain (encephalitis) or muscle (myositis, rhabdomyolysis) tissues, and multi-organ failure (for example, respiratory and kidney failure). Flu virus infection of the respiratory tract can trigger an extreme inflammatory response in the body and can lead to sepsis, the body’s life-threatening response to infection. Flu also can make chronic medical problems worse. For example, people with asthma may experience asthma attacks while they have flu, and people with chronic heart disease may experience a worsening of this condition triggered by flu.
INFLUENZA (FLU) VACCINE
What is flu shot?
Influenza (flu) shot is a flu vaccine given with a needle, usually in the arm. Seasonal flu shots protect against the three or four influenza viruses that research suggests may be most common during the upcoming season.
The composition of flu vaccines has been updated for the 2020-2021 flu season.
Is there more than one type of flu shot available?
Yes. There are different flu vaccine manufacturers and multiple influenza vaccine products licensed and recommended for use in the United States.
CDC recommends the use of any licensed, age-appropriate influenza vaccine during the 2020-2021 influenza season, including inactivated influenza vaccine [IIV], recombinant influenza vaccine [RIV], or live attenuated influenza vaccine (LAIV). No preference is expressed for any influenza vaccine over another. Both trivalent (three-ingredient) and quadrivalent (four-ingredient) influenza vaccines will be available.
Trivalent flu vaccines include:


A trivalent flu shot made using an adjuvant (an ingredient that helps create a stronger immune response), approved for people 65 years of age and older.
Quadrivalent flu vaccines include:
Standard-dose quadrivalent influenza shots that are manufactured using virus grown in eggs. These include QUADRIFLU 4, FLUARIX TETRA, INFLUVAC TETRA, and VAXIFLU 4 in India. Different influenza shots are licensed for different age groups. These four vaccines are approved for people 6 months of age and older. Most influenza shots are given in an arm muscle with a needle.
Each of these vaccines is prepared based on Northern Hemisphere (NH) and Southern Hemisphere (SH).
Preventing influenza disease is challenging, as influenza virus is characterized by frequent mutations due to antigenic drifts and antigenic shifts. To ensure optimal vaccine efficacy against prevailing strains, the antigenic composition of the vaccine is revised twice annually in both the northern hemisphere (NH) and southern hemisphere (SH), and adjusted to the antigenic characteristics of the circulating viruses obtained within the WHO Global Influenza Surveillance and Response System (GISRS). This allows the vaccine manufacturers 4-6 months to manufacture the vaccine for the specific hemisphere.
As per WHO, India is categorized in SH tropical Asia vaccination zone. The vaccine strains may be similar for both hemisphere formulations or different depending on the circulating strains. If the composition of both the hemisphere formulations is the same, as happened in 2017, one can use any of the latest available vaccines from either hemisphere. In India, both NH and SH influenza vaccines are available.
For this season, WHO has recommended influenza vaccines with different strains for the NH and SH. At present, the influenza vaccine meant for those living in the northern hemisphere is available and being used in India. WHO recommendations on vaccine formulation for India strongly favor the Southern hemisphere vaccine rather than the one for the Northern hemisphere. Not many pediatricians are aware of these facts and continue to prescribe the available vaccine without going through the details. Prescribing the currently available NH vaccine in India is not scientifically correct and will not serve the purpose of vaccination. There is a need to educate not only the parents but also the pediatricians about using the appropriate influenza vaccine and prescribe it after verifying the scientific facts rather than use whatever is available.
The common formulation used for each vaccine in 0.5ml dose of Southern Hemisphere strain* are:
A/Victoria/2570/2019 (H1N1)pdm09 -like strain (IVR 215) ……..15microgram HA**
A/Hong Kong/2671/2019 (H3N2) -like strain (NIB 121) ……..15microgram HA**
B/Washington/02/2019 -like strain (wild type) ……..15microgram HA**
B/Phuket/3073/2013 -like strain (wild type) ……..15microgram HA**
*propagated in fertilized hen’s eggs from healthy chicken flocks
**hemagglutinin
Each brand of influenza (flu) vaccine has different adjuvants depending on the company.
All are split influenza vaccines. All are egg inoculated vaccine so are contraindicated to those having egg allergy. In India, no nasal vaccine or vaccine made by recombinant technique or subunit vaccine are not available till now.
a) Vaxiflu 4
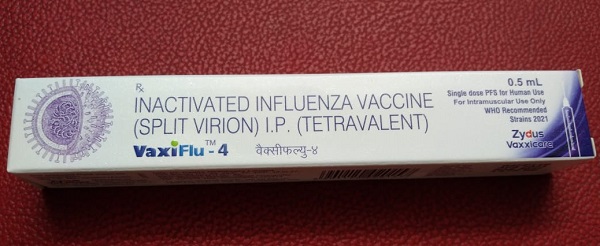

Vaxiflu 4 is a quadrivalent vaccine with a fixed dose of 0.5ml. It belongs to the company Zydus (Made in India). It is a quadrivalent vaccine against common influenza strains present in India.
Vaxiflu 4 (Influenza/ Flu) Vaccine Price in India: [Click here]
b) Quadriflu 4


Quadriflu, also known as FluQuadri is prepared by Sanofi, a quadrivalent vaccine against common influenza strains present in India. It comes as Quadriflu JR & Quadriflu SR. JR is for children from 6 months to 3 years of age. It is an 0.25ml dose vaccine with lower antigenic strength for younger children. While SR vaccine is for all age groups above 3 years of age. It is an 0.5ml dose vaccine.
Quadriflu (Influenza/ Flu) Vaccine Price in India: [Click here]
c) Influvac Tetra
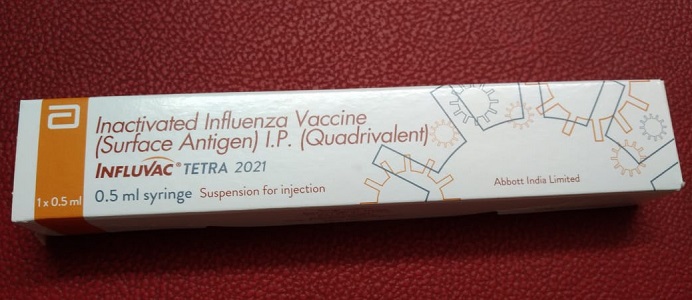

Influvac Tetra is a quadrivalent vaccine with a fixed dose of 0.5ml, belongs to the company Abbott. Influvac tetra comes as a prefilled syringe.
Influvac Tetra (Influenza/ Flu) Vaccine Price in India: [Click here]
d) Fluarix Tetra

Fluarix Tetra is a quadrivalent vaccine with a fixed dose of 0.5ml. It belongs to the company GSK. It is a quadrivalent vaccine against common influenza strains present in India.
Fluarix Tetra (Influenza/ Flu) Vaccine Price in India: [Click here]
There are many flu vaccine options to choose from, but the most important thing is for all people 6 months and older to get a flu vaccine every year. If you have questions about which vaccine is best for you, talk to your doctor or other health care professional. More information on approved flu vaccines for the flu season, and age indications for each vaccine can be obtained from Dr. Vinit Mehta [AASHREY CHILD CLINIC (Child Specialist/ Pediatrician in Vadodara) Vaccination Centre]
How effective is the seasonal flu shot?
Influenza (flu) vaccine effectiveness (VE) can vary. The protection provided by a flu vaccine varies from season to season and depends in part on the age and health status of the person getting the vaccine and the similarity or “match” between the viruses in the vaccine and those in circulation. During years when the flu vaccine match is good, the benefits of flu vaccination will vary, depending on factors like the characteristics of the person being vaccinated (for example, their health and age), what influenza viruses are circulating that season and, potentially, which type of flu vaccine was used. For more information, see Vaccine Effectiveness – How well does the Flu Vaccine Work.
Does a flu vaccine increase your risk of getting COVID-19?
There is no evidence that getting a flu vaccine increases your risk of getting sick from a coronavirus, like the one that causes COVID-19.
The Canadian findings highlighted the protective benefits of flu vaccination.
Also, according to the current situation as of now (July 2021) in India, covid vaccination has not been started for children below 18 years of age. It is under trial and when the vaccination will start is not clear. Till then it is advisable to go for influenza (flu) vaccine for children, due to the following reasons:
- Flu and COVID19 have similar presentations
- Until now, Flu has proven to be more complicated in children than COVID19. There are several hospital admissions and death in children due to flu every year in India. While COVID19 virus does not affect children as there are no ACE receptors in children.
- Also, there is a panic among parents that the next wave of COVID19 is going to affect children. So Flu also can present with similar symptoms of fever, cough, or cold, so instead of panicking about COVID19 infection while the child is having Influenza (flu) infection, it is better to get vaccinated with Influenza (flu) vaccine.
Can I get seasonal flu even though I got a flu vaccine this year?
Yes. It’s possible to get sick with flu even if you have been vaccinated (although you won’t know for sure unless you get a flu test). This is possible for the following reasons:
- You may be exposed to a flu virus shortly before getting vaccinated or during the period that it takes the body to gain protection after getting vaccinated. This exposure may result in you becoming ill with flu before the vaccine begins to protect you. (Antibodies that provide protection develop in the body about 2 weeks after vaccination.)
- You may be exposed to a flu virus that is not included in the seasonal flu vaccine. There are many different flu viruses that circulate every year. A flu vaccine is made to protect against the three or four flu viruses that research suggests will be most common.
- Unfortunately, some people can become infected with a flu virus a flu vaccine is designed to protect against, despite getting vaccinated. The protection provided by flu vaccination can vary widely, based in part on the health and age factors of the person getting vaccinated. In general, a flu vaccine works best among healthy younger adults and older children. Some older people and people with certain chronic illnesses may develop less immunity after vaccination. Flu vaccination is not a perfect tool, but it is the best way to protect against flu infection.
What protection does a flu vaccine provide if I do get sick with flu?
Some people who get vaccinated may still get sick. However, flu vaccination has been shown in some studies to reduce severity of illness in people who get vaccinated but still get sick. A 2017 study showed that flu vaccination reduced deaths, intensive care unit (ICU) admissions, ICU length of stay, and overall duration of hospitalization among hospitalized adults with flu. Another study in 2018 showed that a vaccinated adult who was hospitalized with flu was 59 percent less likely to be admitted to the ICU than someone who had not been vaccinated. Among adults in the ICU with flu, vaccinated patients on average spent 4 fewer days in the hospital than those who were not vaccinated.
Influenza (Flu) Vaccine Benefits
What are the benefits of flu vaccination?


There are many reasons to get the influenza (flu) vaccine each year. Because of the ongoing COVID-19 pandemic, getting a flu vaccine during 2020-2021 will be more important than ever. Flu vaccines will not prevent COVID-19, but they will reduce the burden of flu illnesses, hospitalizations, and deaths on the health care system and conserve scarce medical resources for the care of people with COVID-19.
Below is a summary of the benefits of flu vaccination and selected scientific studies that support these benefits.
- Flu vaccination can keep you from getting sick with the flu.
-
- Flu vaccine prevents millions of illnesses and flu-related doctor’s visits each year. For example, during 2019-2020, flu vaccination prevented an estimated 7.5 million influenza illnesses, 3.7 million influenza-associated medical visits, 105,000 influenza-associated hospitalizations, and 6,300 influenza-associated deaths, in USA.
- During seasons when the flu vaccine viruses are similar to circulating flu viruses, flu vaccine has been shown to reduce the risk of having to go to the doctor with flu by 40 percent to 60 percent.
- Flu vaccination can reduce the risk of flu-associated hospitalization for children, working-age adults, and older adults.
-
- Flu vaccine prevents tens of thousands of hospitalizations each year. For example, during 2019-2020 flu vaccination prevented an estimated 105,000 flu-related hospitalizations.
- A 2014 studyexternal icon showed that the flu vaccine reduced children’s risk of flu-related pediatric intensive care unit (PICU) admission by 74% during flu seasons from 2010-2012.
- In recent years, flu vaccines have reduced the risk of flu-associated hospitalizations among older adultsexternal icon on average by about 40%.
-
- A 2018 study showed that from 2012 to 2015, flu vaccination among adults reduced the risk of being admitted to an intensive care unit (ICU) with flu by 82 percent.
- Flu vaccination is an important preventive tool for people with chronic health conditions.
-
- Flu vaccination has been associated with lower rates of some cardiac events among people with heart disease, especially among those who had had a cardiac event in the past year.
- Flu vaccination can reduce worsening and hospitalization for flu-related chronic lung disease, such as in persons with chronic obstructive pulmonary disease (COPD).
- Flu vaccination also has been shown in separate studies to be associated with reduced hospitalizations among people with diabetes and chronic lung disease.
- Many people at higher risk from flu also seem to be at higher risk from COVID-19.
- Flu vaccination helps protect women during and after pregnancy.
-
- Vaccination reduces the risk of flu-associated acute respiratory infection in pregnant women by about one-half.
- A 2018 study that included influenza seasons from 2010-2016 showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40 percent.
- A number of studies have shown that in addition to helping to protect pregnant women, a flu vaccine given during pregnancy helps protect the baby from flu for several months after birth when he or she is not old enough to be vaccinated.
- Flu vaccines can be lifesaving in children.
-
- A 2017 study was the first of its kind to show that flu vaccination can significantly reduce a child’s risk of dying from flu.
- Flu vaccination has been shown in several studies to reduce severity of illness in people who get vaccinated but still get sick.
-
- A 2017 study showed that flu vaccination reduced deaths, intensive care unit (ICU) admissions, ICU length of stay, and overall duration of hospitalization among hospitalized flu patients.
- A 2018 study showed that among adults hospitalized with flu, vaccinated patients were 59 percent less likely to be admitted to the ICU than those who had not been vaccinated. Among adults in the ICU with flu, vaccinated patients on average spent 4 fewer days in the hospital than those who were not vaccinated.
- Getting vaccinated yourself may also protect people around you, including those who are more vulnerable to serious flu illness, like babies and young children, older people, and people with certain chronic health conditions.
*References for the studies listed above can be found at Publications on Influenza Vaccine Benefits. Also, see the A Strong Defense Against Flu: Get Vaccinated! fact sheet.
Influenza (Flu) Vaccine Match
What is meant by a “good match” between viruses in the vaccine and circulating influenza viruses?
A “good match” is said to occur when the flu vaccine viruses are used to produce flu vaccine and the viruses circulating among people during a given influenza season are “like” one another such that the antibodies produced by vaccination protect against infection with circulating viruses.
What if circulating viruses and vaccine viruses are different?
During seasons when one or more of the circulating viruses are different or “drifted” from the vaccine viruses, vaccine effectiveness against the drifted viruses can be reduced. It’s important to remember that the flu vaccine protects against three or four different flu viruses and multiple viruses usually circulate during any one season. Even if the effectiveness of the vaccine is reduced against one virus it can still be effective at preventing flu illness caused by the other circulating viruses. For these reasons, CDC continues to recommend flu vaccination for everyone 6 months and older even if vaccine effectiveness against one or more viruses is reduced.
Why is there sometimes not a good match between a vaccine virus and circulating viruses?
Flu viruses are constantly changing (called “antigenic drift”) – they can change from one season to the next or they can even change within the course of one flu season. Experts must pick which viruses to include in the vaccine many months in advance in order for the vaccine to be produced and delivered on time. (For more information about the vaccine virus selection process visit Selecting the Viruses in the Influenza (Flu) Vaccine.) Because of these factors, there is always the possibility of a less than optimal match between circulating viruses and the viruses used to produce vaccines.
The production process for some seasonal vaccines also may impact how well vaccine works against certain viruses, especially influenza A (H3N2) viruses. Growth in eggs is part of the production process for most seasonal flu vaccines. While all influenza viruses undergo changes when they are grown in eggs, changes in influenza A(H3N2) viruses are more likely to result in antigenic changes compared with changes in other influenza viruses. These so-called “egg-adapted changes” are present in most of the vaccine viruses recommended for use in egg-based vaccine production and may reduce their potential effectiveness against circulating influenza viruses. Advances in vaccine production technologies (for example, cell-based and recombinant technology) and advanced molecular techniques are being explored as ways to improve flu vaccine effectiveness. Learn more by visiting, Advancements in Influenza Vaccines.
Will this season’s vaccine be a good match for circulating viruses?
It’s not possible to predict with certainty if a flu vaccine will be like circulating flu viruses because flu viruses are constantly changing. A flu vaccine is made to protect against the flu viruses that research and surveillance indicate will likely be most common during the season. Over the course of flu season, CDC studies samples of circulating flu viruses to evaluate how close a match there is between viruses used to make the flu vaccine and circulating flu viruses. For more details contact your pediatrician or book a consult now.
Influenza (Flu) Vaccine Side-effects (What to Expect)
Can a flu vaccine give me flu?
No, a flu vaccine cannot cause flu illness. Flu vaccines that are administered with a needle (flu shots) are currently made in two ways: the vaccine is made either with a) flu vaccine viruses that have been killed (inactivated) and are therefore not infectious, or b) with proteins from a flu vaccine virus instead of flu vaccine viruses (which is the case for recombinant influenza vaccine). Nasal spray vaccine is made with attenuated (weakened) live flu viruses, and also cannot cause flu illness. The weakened viruses are cold-adapted, which means they are designed to only cause infection at the cooler temperatures found within the nose. The viruses cannot infect the lungs or other areas where warmer temperatures exist.
What side effects can occur after getting a flu vaccine?
While a flu vaccine cannot give you flu illness, there are different side effects that may be associated with getting a flu shot or a nasal spray flu vaccine. These side effects are mild and short-lasting, especially when compared to symptoms of a bad cases of flu.
A flu shot: The viruses in a flu shot are killed (inactivated), so you cannot get flu from a flu shot. Some minor side effects that may occur are:
- Soreness, redness, and/or swelling where the shot was given
- Headache (low grade)
- Fever
- Muscle aches
- Nausea
- Fatigue
The nasal spray: The viruses in the nasal spray vaccine are weakened and do not cause severe symptoms often associated with influenza illness. In children, side effects from the nasal spray may include:
- Runny nose
- Wheezing
- Headache
- Vomiting
- Muscle aches
- Fever (low grade)
In adults, side effects from the nasal spray vaccine may include:
- Runny nose
- Headache
- Sore throat
- Cough
If these problems occur, they begin soon after vaccination and usually are mild and short-lived. A flu shot, like other injections, can occasionally cause fainting. Tell your provider if you feel dizzy or have vision changes or ringing in the ears. As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injuries, or death.
Can severe problems occur with Influenza (flu) vaccine?
Life-threatening allergic reactions to flu shots are very rare. Signs of a serious allergic reaction can include breathing problems, hoarseness or wheezing, hives, paleness, weakness, a fast heartbeat, or dizziness. If they do occur, it is usually within a few minutes to a few hours after receiving the shot. These reactions can occur among persons who are allergic to something that is in the vaccine, such as egg protein or other ingredients. While severe reactions are uncommon, you should let your doctor, nurse, clinic, or pharmacist know if you have a history of allergy or severe reaction to influenza vaccine or any part of flu vaccine.
There is a small possibility that flu vaccine could be associated with Guillain-Barré syndrome, generally, no more than 1 or 2 cases per million people vaccinated. This is much lower than the risk of severe complications from flu, which can be prevented by flu vaccine.
What should I do if I have had a serious reaction to the seasonal flu vaccine?
Call a doctor or get to a doctor right away.
Tell your doctor what happened, the date and time it happened, and when you got the flu shot.
Ask your doctor, nurse, or health department to file a Vaccine Adverse Event Reporting System. Reports are welcome from all concerned individuals: patients, parents, health care providers, pharmacists and vaccine manufacturers.
Why do some people not feel well after getting a flu shot?
Flu vaccine side effects are generally mild and go away on their own within a few days. Some side effects that may occur from a flu shot include soreness, redness, and/or swelling where the shot was given, headache (low grade), fever, nausea, muscle aches, and fatigue. The flu shot, like other injections, can occasionally cause fainting.
What about people who get a seasonal flu vaccine and still get sick with flu symptoms?
There are several reasons why someone might get flu symptoms, even after they have been vaccinated against flu.
- One reason is that some people can become ill from other respiratory viruses besides flu such as rhinoviruses, which are associated with the common cold, cause symptoms similar to flu, and also spread and cause illness during the flu season. The flu vaccine only protects against flu, not other illnesses.
- Another explanation is that it is possible to be exposed to flu viruses, which cause flu, shortly before getting vaccinated or during the two-week period after vaccination that it takes the body to develop immune protection. This exposure may result in a person becoming ill with flu before protection from vaccination takes effect.
- A third reason why some people may experience flu symptoms despite getting vaccinated is that they may have been exposed to a flu virus that is very different from the viruses the vaccine is designed to protect against. The ability of a flu vaccine to protect a person depends largely on the similarity or “match” between the viruses selected to make the vaccine and those spreading and causing illness. There are many different flu viruses that spread and cause illness among people.
- The final explanation for experiencing flu symptoms after vaccination is that flu vaccines vary in how well they work and some people who get vaccinated still get sick. When that happens, though vaccination has been shown in several studies to reduce the severity of illness in those people who get vaccinated but still get sick.
What protection does a flu vaccine provide if I do get sick with flu?
Some people who get vaccinated may still get sick. However, flu vaccination has been shown in several studies to reduce the severity of illness in people who get vaccinated but still get sick:
- A 2017 study showed that flu vaccination reduced deaths, intensive care unit (ICU) admissions, ICU length of stay, and overall duration of hospitalization among hospitalized flu patients.
- Another study in 2018showed that a vaccinated adult who was hospitalized with flu was 59% less likely to be admitted to an intensive care unit (ICU) than someone who had not been vaccinated. Among adults in the ICU with flu, vaccinated patients on average spent 4 fewer days in the hospital than those who were not vaccinated.
In addition, it’s important to remember that the flu vaccine protects against three or four different viruses and multiple viruses usually circulate during any one season. For these reasons, CDC continues to recommend flu vaccination for everyone 6 months and older even if vaccine effectiveness against one or more viruses is reduced.
Special Consideration Regarding Egg Allergy
People with egg allergies can receive any licensed, recommended age-appropriate influenza vaccine (IIV, RIV4, or LAIV4) that is otherwise appropriate. People who have a history of severe egg allergy (those who have had any symptom other than hives after exposure to egg) should be vaccinated in a medical setting, supervised by a health care provider who is able to recognize and manage severe allergic reactions. Two completely egg-free (ovalbumin-free) flu vaccine options are available: quadrivalent recombinant vaccine and quadrivalent cell-based vaccine.
Conclusion
Influenza has been responsible for pandemics several times. It has been responsible for several hospital admissions, ICU admissions, and death, especially in children. A vaccine is available for influenza (flu), so it is better to get your family vaccinated against flu. Parents can also be a spreader of flu in the family, neighborhood and at the workplace, so it is always better to take your yearly flu shot. If cost is an issue for this yearly flu vaccination then high-risk groups especially children below 8 years, pregnant females, the elderly, individuals with chronic health conditions, etc should be vaccinated at least. So book a consult with your doctor now for your yearly flu shot.


